Abstract
Following the detection of the new coronavirus1 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its spread outside of China, Europe has experienced large epidemics of coronavirus disease 2019 (COVID-19). In response, many European countries have implemented non-pharmaceutical interventions, such as the closure of schools and national lockdowns. Here we study the effect of major interventions across 11 European countries for the period from the start of the COVID-19 epidemics in February 2020 until 4 May 2020, when lockdowns started to be lifted. Our model calculates backwards from observed deaths to estimate transmission that occurred several weeks previously, allowing for the time lag between infection and death. We use partial pooling of information between countries, with both individual and shared effects on the time-varying reproduction number (Rt). Pooling allows for more information to be used, helps to overcome idiosyncrasies in the data and enables more-timely estimates. Our model relies on fixed estimates of some epidemiological parameters (such as the infection fatality rate), does not include importation or subnational variation and assumes that changes in Rt are an immediate response to interventions rather than gradual changes in behaviour. Amidst the ongoing pandemic, we rely on death data that are incomplete, show systematic biases in reporting and are subject to future consolidation. We estimate that—for all of the countries we consider here—current interventions have been sufficient to drive Rt below 1 (probability Rt < 1.0 is greater than 99%) and achieve control of the epidemic. We estimate that across all 11 countries combined, between 12 and 15 million individuals were infected with SARS-CoV-2 up to 4 May 2020, representing between 3.2% and 4.0% of the population. Our results show that major non-pharmaceutical interventions—and lockdowns in particular—have had a large effect on reducing transmission. Continued intervention should be considered to keep transmission of SARS-CoV-2 under control.
Similar content being viewed by others
Main
Following the identification of the new coronavirus SARS-CoV-2 in Wuhan (China) in December 2019 and its global spread, large epidemics of COVID-19 have ensued in Europe. In response to the rising numbers of cases and deaths and to preserve health systems, European countries—as with those in Asia—have implemented measures to control their epidemics. These large-scale non-pharmaceutical interventions vary between countries, but include social distancing (such as banning large gatherings), border closures, school closures, measures to isolate symptomatic individuals and their contacts, and large-scale lockdowns of populations with all but essential internal travel banned. Understanding whether these interventions have had the desired effect of controlling the epidemic, and which interventions are necessary to maintain control, is critical given their large economic and social costs. The key aim of these interventions is to reduce Rt, a fundamental epidemiological quantity that represents the average number of infections generated at time t by each infected case over the course of their infection.
In China, strict movement restrictions and other measures (including case isolation and quarantine) began to be introduced from 23 January 2020, which achieved a downward trend in the number of confirmed new cases during February and resulted in zero new confirmed indigenous cases in Wuhan by 19 March 2020. Studies have estimated how the values of Rt changed during this time in different areas of China, from around 2–4 during the uncontrolled epidemic to below 1 (refs. 1,2).
Estimating Rt for SARS-CoV-2 presents challenges, owing to the high proportion of infections that are not detected by health systems1,3,4 and to the regular changes in testing policies, which resulted in different proportions of infections being detected over time and between countries. Initially, most countries had the capacity to test only a small proportion of suspected cases and reserved tests for severely ill patients or for high-risk groups (for example, the contacts of positively tested individuals).
An alternative way to estimate the course of the epidemic is to calculate backwards from observed deaths to the number of infections. We introduce a Bayesian mechanistic model linking the infection cycle to observed deaths, inferring the total population infected (attack rates) as well as Rt. We assess whether there is evidence that interventions have so far been successful at reducing Rt to values below 1. We simulate a hypothetical counterfactual scenario in which Rt remains at starting levels to estimate the deaths that would have occurred without interventions.
Reported deaths are likely to be far more reliable than case data—although reported death data still have limitations. First, early deaths attributable to COVID-19 may have been missed. Second, there is variation in the reporting of deaths by country and over time. Third, reporting delays are expected and can be both systematic and random in nature. We attempt to overcome these data limitations by using a consolidated data source, incorporating noise in our observational model, partially pooling of information between countries and performing a sensitivity analysis under scenarios of underreporting to test our conclusions (Supplementary Information).
Our model relies on fixed estimates of some epidemiological parameters, such as the onset-to-death distribution, the infection fatality rate and the generation distribution, that are based on previous work5,6; we perform a sensitivity analysis on these parameters. Our parametric form of Rt assumes that changes in Rt are an immediate response to interventions rather than gradual changes in behaviour, and it does not include importation or subnational variation. We assume that individual interventions have a similar effect in different countries, and that the efficacy of these interventions remains constant over time. Our framework infers Rt from mortality data, while accounting for time lags since infections occurred. As a result, even with perfect data and partial pooling, we cannot perfectly predict the current value of Rt. However, the credible intervals on Rt show the self-consistent behaviour that is a hallmark of a fully Bayesian analysis throughout the entire period we study, exhibiting appropriate shrinkage as more data become available (Supplementary Videos 1–3).
Italy was the first European country to begin major non-pharmaceutical interventions, and other countries followed soon afterwards (Extended Data Fig. 4). The onset of interventions ranged between 2 March and 29 March 2020. We analysed data on mortality from COVID-19 in 11 European countries until 4 May 2020, at which point lockdowns were relaxed in Italy and Spain. For each country, we model the number of infections, the number of deaths and Rt (Fig. 1). Rt is modelled as a piecewise constant function that changes only when an intervention occurs. Each country has its own individual starting Rt before interventions took place. For all countries, interventions are assumed to have the same relative effect on Rt and are informed by mortality data across all countries. The only exception is that we use partial pooling to introduce country-specific effects of the effectiveness of the last intervention introduced in the study period in a country (which is usually lockdown).
Left, daily number of infections. Brown bars are reported infections; blue bands are predicted infections; dark blue, 50% credible interval; light blue 95% credible interval. The number of daily infections estimated by our model drops immediately after an intervention, as we assume that all infected people become immediately less infectious through the intervention. Afterwards, if Rt is above 1, the number of infections will start growing again. Middle, daily number of deaths. Brown bars are reported deaths; blue bands are predicted deaths; credible intervals are as in the left plot. Right, Rt. Dark green, 50% credible interval; light green, 95% credible interval. Icons are interventions, shown at the time at which they occurred.
Estimated infections, R t and effect sizes
In all countries, we estimate there are orders-of-magnitude fewer infections detected (Fig. 1, Extended Data Figs. 1, 2) than true infections, most likely owing to mild and asymptomatic infections as well as limited testing capacities and changes in testing policy. In Italy, our results suggest that—cumulatively—2.8 (2.2–3.5) million people (all parenthetical ranges refer to 95% credible intervals) have been infected as of 4 May 2020, which gives an attack rate of 4.6% (3.6–5.8%) of the population (Table 1). In Spain (which has also experienced a large number of deaths), we estimate that 5.5% of the population (2.6 (2.1–3.3) million people) have been infected to date. Germany, the most populous country in our study, is estimated to have one of the lowest attack rates at 0.85% with 710,000 (550,000–930,000) people infected. Belgium has the highest estimated attack rates of 8%, followed by Spain with 5.5%. Although there have been few reliable national serological studies7, initial small-scale surveys in Austria8 and Denmark9 closely align with our estimates. A much larger study in Spain is very closely aligned with our estimates10. To some extent, these initial results validate our choice of infection fatality rate.
Averaged across all countries, we estimate the initial Rt to be 3.8 (2.4–5.6), consistent with previous analyses1,11. These estimates are informed by our choice of generation-interval distribution and the initial growth rate of observed deaths. A shorter assumed generation time results in lower starting Rt (Supplementary Discussion 3). The initial values of Rt are also uncertain, owing to (a) importation (rather than local transmission) being the dominant source of new infections in the early period of the epidemic and (b) possible under-ascertainment in deaths, particularly before testing became widespread. We perform sensitivity analyses around these parameters (Supplementary Discussions 10, 11).
We estimate large reductions in Rt in response to the combined non-pharmaceutical interventions. Our results—which are driven more by countries with advanced epidemics and larger numbers of deaths—suggest that these interventions have together had a substantial effect on transmission, as measured by changes in the estimated Rt. At the time of this study, we find current estimates of Rt to range from a posterior mean of 0.44 (0.26–0.61) for Norway to a posterior mean of 0.82 (0.73–0.93) for Belgium, with an average of 0.66 across the 11 countries—an 82% reduction compared to the pre-intervention values. For all countries, we find that current interventions have been sufficient to drive Rt below 1 (probability Rt < 1.0 is greater than 99% across all countries we consider) and achieve control of the epidemic. These conclusions are corroborated by studies from individual countries—France12, Spain13, Germany14 and the UK15—over a similar period, which arrive at very similar estimates despite different methodologies and data. For example, a previous study12 estimates an Rt of 0.67 for France using hospitalization records (we estimate 0.68); for Germany, the Robert Koch Institute reports Rt of 0.76 using electronically notified cases14 (we estimate 0.71). The retrospective stability of our model (Supplementary Videos 1–3) is variable when the implementations of interventions are very dissimilar; an example of this is seen in Sweden, where interventions were dissimilar to other countries and led initially to large uncertainty. Our model uncertainty is also dependent on the magnitude of Rt; this occurs because infections are a nonlinear function of Rt and are sensitive to small increases. Uncertainty shrinks greatly when Rt is reduced. Examples of this effect are seen in all countries, but it is most pronounced in Belgium and France; these countries show large uncertainties in the number of infections in the early period of the epidemic. Our choice of parameterizing Rt using piecewise constant functions means that we cannot capture the fine-scale variation that could be achieved by using additional covariates.
Lockdown has an identifiable large effect on transmission (81% (75–87%) reduction) (Fig. 2). The close spacing of interventions in time (Extended Data Fig. 4) means that the individual effects of the other interventions are not identifiable (Fig. 2). Our partial pooling model requires only one country to provide a signal for the effect of a given intervention, and this effect is then shared across all countries. Although this sharing can potentially lead to initial over- or under-estimation of the effect of an intervention, it also means that a consistent signal for all countries can be estimated before that signal is present in data from an individual country16. Therefore, this sharing is potentially useful for generating early warnings, by leveraging what happened in countries with earlier epidemics to inform countries with more-recent epidemics.
Our model includes five covariates for governmental interventions, adjusting for whether the intervention was the first one undertaken by the government in response to COVID-19 (red) or was subsequent to other interventions (green). Mean relative percentage reduction in Rt is shown with 95% posterior credible intervals. If 100% reduction is achieved, Rt = 0 and there is no more transmission of COVID-19. Lockdown is significantly different from the other interventions; the other interventions are not significantly different from each other, probably owing to the fact that many interventions occurred on the same day or within days of each other (as shown in Extended Data Fig. 4). Results are derived from a model that represents 11 countries with a total population of 375 million and 128,928 reported COVID-19-related deaths up to 4 May 2020.
Estimated effect of interventions on deaths
Extended Data Table 1 shows total deaths forecast from the beginning of the epidemic up to and including 4 May 2020 under our fitted model and under the counterfactual model, which predicts what would have happened if no interventions were implemented (and Rt = R0; that is, the initial Rt estimated before interventions came into effect).
By comparing the deaths predicted under the model with no interventions to the deaths predicted in our intervention model, we calculated the total deaths averted in our study period. We find that across 11 countries 3.1 (2.8–3.5) million deaths have been averted owing to interventions since the beginning of the epidemic; Extended Data Fig. 5 compares the actual total deaths to the counterfactual total deaths. The counterfactual model without interventions is illustrative only, and reflects the assumptions of our model. We do not account for changes in behaviour; in reality, even in the absence of government interventions we would expect Rt to decrease and therefore would overestimate deaths in the no-intervention model. Conversely, we do not consider the effect on the infection fatality rate as a result of an overwhelmed health system in which patients may not be able to access critical care facilities, which would underestimate the number of counterfactual deaths. In the Supplementary Information, we show further counterfactual estimates under differing assumptions of the generation distribution and onset-to-death distribution and all scenarios broadly show the same trends. Given this agreement across differing scenarios, we believe our estimates for the counterfactual deaths averted to be plausible.
Discussion
During the ongoing transmission of SARS-CoV-2 in Europe, we analyse trends in the numbers of deaths to assess the extent to which transmission has been reduced. Representing the infection process associated with COVID-19 using a semi-mechanistic, joint Bayesian hierarchical model, we can reproduce trends observed in the data relating to deaths and produce empirically driven predictions that are valid over short time horizons.
We estimate that there have been many more infections than are currently reported. The high level of under-ascertainment of infections that we estimate here is probably due to the focus on testing in hospital settings, which misses milder or asymptomatic cases in the community. Despite this, we estimate that only a relatively small minority of individuals in each country have been infected (Table 1). Our estimates imply that the populations in Europe are not close to herd immunity (about 70% if R0 is 3.8)17. Furthermore, with values of Rt below 1 in all countries, the rate of acquisition of herd immunity will slow down rapidly. Our estimates for attack rates during our study period are consistent with those reported from national serological studies7. Similarly, comparable studies estimating Rt all agree that the number as of 4 May 2020 is less than 1.
To our knowledge, our modelling approach is unique in pooling information from multiple countries at once. Using this approach means that we require a central consolidated data source (such as data from the European Centre of Disease Control (ECDC)), and also that trends in some countries will be affected by those countries with more data. We argue that this effect is beneficial, in that it helps to minimize idiosyncrasies in the data16, as well as to improve consistency of estimates over time. Although our qualitative conclusions surrounding the effect of interventions and the finding that Rt is less than 1 are robust to our choice of whether to incorporate pooling or not, the ability to use a greater extent of available data and share information across countries in a statistically principled manner markedly improves the consistency of model predictions across the study period (Supplementary Videos 1–3).
Most interventions were implemented in rapid succession in many countries, and as such it is difficult to disentangle the individual effect sizes of each intervention. In our analysis, we find that only the effect of lockdown is identifiable, and that it has a substantial effect (81% (75–87%) reduction in Rt). Taking into account country-specific effects, the effect size of lockdown remains large across all countries (Supplementary Fig. 29).
We acknowledge the limitations of existing mortality data relating to COVID-19—in particular, deaths outside hospitals may be underreported. However, by using the ECDC data, we rely on a comprehensive data source that is refined and updated each day in a systematic process. Our sensitivity analysis of underreporting and statistical-measurement noise suggests that we may slightly underestimate the attack rates in some countries, but this does not change our overall conclusions pertaining to Rt. However, even if the data were complete, our method cannot surmount the time lag between infections and deaths and can only fully identify trends in infections 2–3 weeks earlier. Extensions of our model could use case, hospitalization or intensive care data, but reconciling the different biases inherent in these sources while ensuring parsimony is challenging and would require additional assumptions.
The modern understanding of infectious disease, combined with a global publicized response, has meant that nationwide interventions could be implemented with widespread adherence and support. Given the observed infection fatality ratios and the epidemiology of COVID-19, major non-pharmaceutical interventions have had an effect in reducing transmission in all of the countries we have considered. In all countries in this study, we find that these interventions have reduced Rt below 1, and have contained their epidemics at the current time. When looking at simplistic counterfactual models over the whole epidemic, the number of potential deaths averted is substantial. We cannot say for certain that the current measures will continue to control the epidemic in Europe; however, if current trends continue there is reason for optimism.
Methods
Data
Our model uses daily consolidated death data from the ECDC for 11 European countries currently experiencing the COVID-19 epidemic: Austria, Belgium, Denmark, France, Germany, Italy, Norway, Spain, Sweden, Switzerland and the UK. The ECDC provides information on confirmed cases and deaths attributable to COVID-19. For population counts, we use the United Nations Population Division age-stratified counts18.
We also catalogue data on the nature and type of major non-pharmaceutical interventions. We looked at the government webpages from each country as well as their official public health webpages to identify the latest advice or laws being issued by the government and public health authorities. We collected the following: school closure ordered; case-based measures; public events banned; social distancing encouraged; lockdown decreed; and the time of the first and last intervention. A full list of the timing of these interventions and the sources we have used is provided in the Supplementary Notes, Supplementary Table 2.
By using the ECDC data, we rely on a consolidated data source compiled by the ECDC, who include many sources of data each day, constantly refining and updating data using a comprehensive and systematic process. However, despite the rigorous protocols, countries may vary in the specifics of the data that they report to the ECDC. For example, there is variation in reporting (that is, community versus hospital) and time lags. Despite these issues, we use ECDC data to ensure as much consistency as possible across all countries.
Model
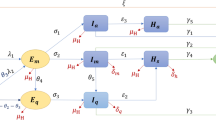
A visual summary of our model is presented in Extended Data Fig. 3; details are provided in the Supplementary Methods.
We fit our model to observed deaths according to ECDC data from 11 European countries. The modelled deaths are informed by an infection-to-death distribution (Supplementary Fig. 1; derived from assumptions about the time from infection to the onset of symptoms and about the time from the onset of symptoms to death), and the population-averaged infection fatality ratio (adjusted for the age structure and contact patterns of each country, as discussed in the Supplementary Methods, Supplementary Table 3).
Given these distributions and ratios, modelled deaths are a function of the number of infections. The number of infections is modelled as the product of Rt with a discrete convolution of the previous infections. Individual components of this convolution sum are weighted by the generation time distribution (the average time from the infection of one person to the time at which they infect another; Supplementary Fig. 2). In our work, we approximate the generation time distribution using the serial interval distribution. Rt is a function of the initial Rt before interventions and the effect sizes from interventions, in which interventions are modelled as piecewise constant functions.
Following the Bayesian hierarchy from bottom to top gives us a full framework to see how interventions affect infections, which can result in deaths. A schematic of our model is shown in Extended Data Fig. 3. To maximize the ability to observe the effect of interventions on deaths, we fit our model jointly for all 11 European countries, and use partial pooling of information between countries with both individual and shared effects on Rt. Partial pooling operates on the last intervention, which is—in most cases—lockdown. The effect of partial pooling can be seen in Supplementary Discussion 12, Supplementary Fig. 29. We chose a balanced prior that encodes the prior belief that interventions have an equal chance of having an effect or not, and ensure a uniform prior on the joint effect of all interventions (Supplementary Fig. 3). We evaluate the effect of our Bayesian prior distribution choices and evaluate our Bayesian posterior calibration to ensure our results are statistically robust.
We perform extensive model validation and sensitivity analyses. We validate our model by cross-validation over a 14-day period (Supplementary Discussion 1, Supplementary Table 1) and we show the fits for holdout samples in Supplementary Figs. 5–15. We check the convergence of the Markov chain Monte Carlo sampler (Supplementary Fig. 4). We consider the sensitivity of our estimates of Rt to the mean of the generation distribution (Supplementary Discussion 3, Supplementary Figs. 16, 17). We further show that the choice of generation distribution does not change our counterfactual conclusions (Supplementary Fig. 18). Using univariate analyses and uninformative priors, we find (Supplementary Fig. 19) that all effects on their own serve to decrease Rt (Supplementary Discussion 4). We compare our model to a non-parametric Gaussian Process model (Supplementary Discussion 5). To assess the effect of individual countries on the results, we perform a ‘leave one country out’ sensitivity analysis (Supplementary Discussion 6, Supplementary Figs. 20, 21). To validate our starting values of Rt, we compare our model against an exponential-growth linear model (Supplementary Discussion 7, Supplementary Fig. 22). Instead of a joint analysis, we consider fits of our model to individual countries (Supplementary Discussion 8, Supplementary Figs. 23–26). We perform a sensitivity analysis with respect to the onset-to-death distribution (Supplementary Discussion 9, Supplementary Fig. 27). We validate our probabilistic seeding scheme through an importance-sampling leave-one-out cross-validation (Supplementary Discussion 10). We consider a model extension with a constant, probabilistic under-reporting (Supplementary Discussion 11), finding that Rt does not change substantially (Supplementary Fig. 28).
Our model is different to other approaches (such as EpiEstim19) that use the discrete renewal equation. We use the renewal equation as a latent process to model infections and propose a generative mechanism to connect these infections to death data. Simply applying the renewal equation directly to death data requires positing a mechanism in which deaths in the past can cause future deaths (see, for example, ref. 20). In addition, for Rt, we are able to use a functional relationship in which non-pharmaceutical interventions can have a direct effect on Rt.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Death counts for the 11 European countries for the time period in our study and the full set of posterior draws from our model are available at https://reshare.ukdataservice.ac.uk/854380/.
Code availability
All source code and data necessary for the replication of our results and figures are available at https://github.com/ImperialCollegeLondon/covid19model. An R package based on our method is available at https://imperialcollegelondon.github.io/epidemia/.
References
Li, R. et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 368, 489–493 (2020).
Zhang, J. et al. Patterns of human social contact and contact with animals in Shanghai, China. Sci. Rep. 9, 15141 (2019).
Zhao, A. J. et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. ciaa344 https://doi.org/10.1093/cid/ciaa344 (2020).
Jombart, T. et al. Inferring the number of COVID-19 cases from recently reported deaths. Wellcome Open Research 5, 78 (2020).
Verity, R. et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet 20, 669–677 (2020).
Bi, Q. et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(20)30287-5 (2020).
Bobrovitz, N. et al. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. Preprint at medRxiv https://doi.org/10.1101/2020.05.10.20097451 (2020).
Statistics Austria. COVID-19 Prevalence Study: Maximum 0.15% of Austrian Population Infected with SARS-CoV-2 (Statistics Austria, 2020).
Erikstrup, C. et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin. Infect. Dis. ciaa849 https://doi.org/10.1093/cid/ciaa849 (2020).
Pollán, M. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet https://doi.org/10.1016/S0140-6736(20)31483-5 (2020).
Zhang, J. et al. Age profile of susceptibility, mixing, and social distancing shape the dynamics of the novel coronavirus disease 2019 outbreak in China. Preprint at medRxiv https://doi.org/10.1101/2020.03.19.20039107 (2020).
Salje, H. et al. Estimating the burden of SARS-CoV-2 in France. Science 369, 208–211 (2020).
Hyafil, A. & Morina, D. Analysis of the impact of lockdown on the reproduction number of the SARS-Cov-2 in Spain. Gac. Sanit. https://doi.org/10.1016/j.gaceta.2020.05.003(2020).
Robert Koch Institute. Coronavirus Disease 2019 (COVID-19) Daily Situation Report of the Robert Koch Institute (Robert Koch Institute, 2020).
Davies, N. G., Kucharski, A. J., Eggo, R. M., Gimma, A. & Edmunds, W. J. The effect of non-pharmaceutical interventions on COVID-19 cases, deaths and demand for hospital services in the UK: a modelling study. Lancet 5, E375–E385 (2020).
Gelman, A. & Hill, J. Data Analysis using Regression and Multilevel/Hierarchical Models (Cambridge Univ. Press, 2006).
Miller, J. C. A note on the derivation of epidemic final sizes. Bull. Math. Biol. 74, 2125–2141 (2012).
United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Data Booklet, ST/ESA/SER.A/424. (United Nations, 2019).
Cori, A., Ferguson, N. M., Fraser, C. & Cauchemez, S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am. J. Epidemiol. 178, 1505–1512 (2013).
Goldstein, E. et al. Reconstructing influenza incidence by deconvolution of daily mortality time series. Proc. Natl Acad. Sci. USA 106, 21825–21829 (2009).
Acknowledgements
S.B. acknowledges the NIHR BRC Imperial College NHS Trust Infection and COVID themes, the Academy of Medical Sciences Springboard award and the Bill and Melinda Gates Foundation. L.C.O. acknowledges funding from a UK Royal Society fellowship. Initial research on covariates in Supplementary Table 2 was crowdsourced; we thank a number of people across the world for help with this. This work was supported by Centre funding from the UK Medical Research Council under a concordat with the UK Department for International Development, the NIHR Health Protection Research Unit in Modelling Methodology and Community Jameel. We thank F. Valka for creating our website, and A. Gelman and the Stan team for helpful discussions. We acknowledge the resources provided by Cirrus UK National Tier-2 HPC Service at EPCC (http://www.cirrus.ac.uk) funded by the University of Edinburgh and EPSRC (EP/P020267/1), and cloud compute time donated by Microsoft and Amazon.
Author information
Authors and Affiliations
Consortia
Contributions
S.B., S.F., S.M. and A.G. conceived and designed the study. S.B., S.F., S.M., A.G., H.C., H.J.T.U., T.A.M., M.A.C.V., J.W.E. and N.M.F. performed analysis. L.C.O., S.B., S.F., A.G., A.C.G., C.A.D., S.R. and N.M.F. wrote the first draft of the paper. S.B., S.F., H.C., C.W., P.W., T.B., P.N.P.G., N.S., L. Cilloni, M.A.C.V. and H.C. collected data. All authors discussed the results and contributed to the revision of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks David Earn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Country-level estimates of infections, deaths and Rt for Belgium, Germany, Sweden and Switzerland.
Left, daily number of infections. Brown bars are reported infections; blue bands are predicted infections; dark blue, 50% credible interval; light blue, 95% credible interval. The number of daily infections estimated by our model drops immediately after an intervention, as we assume that all infected people become immediately less infectious through the intervention. Afterwards, if Rt is above 1, the number of infections will starts growing again. Middle, daily number of deaths. Brown bars are reported deaths; blue bands are predicted deaths; credible intervals are as in the left plot. Right, Rt. Dark green, 50% credible interval; light green, 95% credible interval. Icons are interventions, shown at the time at which they occurred.
Extended Data Fig. 2 Country-level estimates of infections, deaths and Rt for Austria, Norway and Denmark.
Left, daily number of infections. Brown bars are reported infections; blue bands are predicted infections; dark blue, 50% credible interval; light blue, 95% credible interval. The number of daily infections estimated by our model drops immediately after an intervention, as we assume that all infected people become immediately less infectious through the intervention. Afterwards, if Rt is above 1, the number of infections will starts growing again. Middle, daily number of deaths. Brown bars are reported deaths; blue bands are predicted deaths; credible intervals are as in the left plot. Right, Rt. Dark green, 50% CI; light green, 95% CI. Icons are interventions, shown at the time at which they occurred.
Extended Data Fig. 3 Model summary.
This figure shows a summary of the components of our model.
Extended Data Fig. 4 Timings of interventions.
Timings of interventions for the 11 European countries included in our analysis. For further details, see the Supplementary Information.
Extended Data Fig. 5 Deaths averted owing to interventions.
Lower and upper credible interval for the cumulative number of deaths for the 11 European countries in our model with interventions (pink) and the no-interventions counterfactual model (blue). Reported deaths are shown as a thick black line.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Discussion 1-12, Supplementary Notes, Supplementary Figures 1-29, Supplementary Tables 1-3 and Supplementary References.
Video 1
Model estimates for France, Italy, Spain, and the United Kingdom fit to successive days of data until the end of the study period. Estimates are refined over time, and as more data becomes available the posterior distribution concentrates.
Video 2
Model estimates for Belgium, Germany, Sweden, and Switzerland fit to successive days of data until the end of the study period. Estimates are refined over time, and as more data becomes available the posterior distribution concentrates.
Video 3
Model estimates for Austria, Denmark, and Norway fit to successive days of data until the end of the study period. Estimates are refined over time, and as more data becomes available the posterior distribution concentrates.
Rights and permissions
About this article
Cite this article
Flaxman, S., Mishra, S., Gandy, A. et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 584, 257–261 (2020). https://doi.org/10.1038/s41586-020-2405-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-020-2405-7
This article is cited by
-
Establishing the effect of COVID-19 lockdown policy on the utilization of facility-based delivery in Kenya: a multi-method study
BMC Health Services Research (2025)
-
The influence of face masks use, immunity boosters and visiting risky places on mental health during the COVID-19 pandemic in Serbia and Republic of Srpska (Bosnia and Herzegovina)
Journal of Health, Population and Nutrition (2025)
-
Modeling-based design of adaptive control strategy for the effective preparation of ‘Disease X’
BMC Medical Informatics and Decision Making (2025)
-
Optimal pandemic control strategies and cost-effectiveness of COVID-19 non-pharmaceutical interventions in the United States
BMC Global and Public Health (2025)
-
Optimizing quarantine in pandemic control: a multi-stage SEIQR modeling approach to COVID-19 transmission dynamics
BMC Infectious Diseases (2025)



M. Lehmann
A couple of comments and questions:
1. Estimated infection fatality rates (IFR) and the underlying methodologies, assumptions, and data sources are central for the results of the model. Why is this critical information only presented in the “supplementary information” section? As a result, the main source (Verity et al, Lancet Infect Dis 2020:20) does not even appear in the literature list of the main article.
2. For estimating IFR, the article uses the approach and numbers presented in Verity et al., correcting for non-uniform attack rates. Verity et al use very early data and analyses from China, from January and February 2020. Specifically, in order to calculate IFR, Verity et al match incidence reported in Wuhan in January 2020 with data on expatriate prevalence from six international repatriating flights on 30 January and 1 February 2020 (!). Do we really not have more recent and better data by now?
3. In a (very brief) passage on page 2, the authors note the role of serological surveys and, in referring to a few of them (from Austria, Denmark, and Spain), note that “these initial results, to some extent (sic!), validate [the] choice of the IFR.” This is remarkably vague -- what does “to some extent” mean? And to what “extent” does it not validate?
4. The choice of the serological studies that are referenced is not explained and seems to be selective. Key studies are missing, including from the countries under investigation, despite them having been widely discussed; for instance Streek at al. (https://www.medrxiv.org/con... ). Streeck et al report an estimated IFR of 0.36%, which does _not_ seem to validate the choice of the IFR in the current paper. The authors may not agree with this and similar findings, but should such findings not at least be mentioned and discussed?
5. The authors also note the limited reliability of most serological surveys, referring to a recent metastudy of serological surveys by Bobrovitz et al. However, the authors do not make an attempt to compare such limitations with any methodological limitations in Verity et al.
6. The choice of the metastudy of serological surveys is again selective; other recent work is not reflected (see https://www.medrxiv.org/con... ). Based on currently available evidence, Oxford University’s Center for Evidence Based Medicine (CEBM), as of 3rd June 2020, estimates COVID-19 IFR somewhere between 0.1% and 0.41 %, which is _not_ in line with the authors’ IFR. Again: the authors may not agree with the results presented by others, but should these results not at least be mentioned and discussed?
John Smith Replied to M. Lehmann
https://www.medrxiv.org/con... - has that ever been peer reviewed ? According to Carl Bergstrom, renowned epidemiological modeller, the author picked the surveys with lowest estimates of IFR available and ignored the rest in that meta study ...
Pragmatist_in_another_guise Replied to John Smith
Ah, meta-analysis - means of constructing silk purses from sows' ears.
bert
The comment marked as spam, actually put forward, according to me, the valid argument (although rudely stated) that the accuracy of the output (conclusions) of any model depends on the model, but also very much on the accuracy of the data put into the model. I believe all scientists would agree with this?
I have some other arguments that would imply that the conclusions of the model used are far from accurate because the data put into the model are inaccurate:
1. Nobody knows how many people are actually infected by the new corona virus.This because relatively few people have been tested because they had none or little symptoms of the disease. This implies that the relation between corona virus infected people and infection fatality rate is just inaccurate. It could be very inaccurate!
2. Nobody knows which "lock-down measure" influenced infection rate.
3. In Sweden, they didn't do the corona measures. People dying because of the virus in Sweden is the same as in other countries.
Pls don't say this is a SPAM
claude Replied to bert
In Sweden, a country with excellent health care, excess mortality has been clearly higher in the past 3 months than to its neighbouring countries (Norway, Denmark, Finland): https://www.euromomo.eu/gra...
bert Replied to claude
It may be true Claude, more people relatively may have died because of corona in Sweden in the last 3 months. But not that many. But the 'curve' (so the number of deaths), shows the same pattern. how is that possible?
claude Replied to bert
Bert, as far as I know, in Sweden public gatherings >50 people are forbidden, senior homes are closed for visitors, university and schools for teens >~15 do home schooling, travel ban is announced. So maybe these measures take effects? This would argue for several parameters instead of a complete lockdown solely though.
mosmosmos Replied to claude
ICL ran the headline on the release of their paper as 'Lockdown and School Closures may have saved 3m lives. They even showed in fig2 that only lockdown had a significant impact of transmissability which doesn't seem to match the outcomes.
The justification for this figure was comparing actual deaths to the counterfactual.
Sweden neither closed most schools, nor locked down, yet while higher than other countries who put in more stringent rules, only saw a death toll of up to 10% of the counterfactual, even if you consider excess mortality.
How the paper arrives at the conclusion that 'lockdown in particular' slowed the transmission when the only country in the paper which didn't lockdown had a similar outcome is beyond me, it actually appears that NPI's up to lockdown saved the overwhelming majority of the lives (against the counterfactual) and lockdown itself had small supplementary impact.
Pragmatist_in_another_guise Replied to bert
In the UK, Sweden, and perhaps elsewhere, there is need to factor in the impact upon death totals of failure to take measures for effective isolation of high risk populations as in care homes. In the UK elderly patients, some very likely to be infectious were dumped back from hospitals into care homes in order to make room for the deluge of people predicted by 'science'.
Marcel Bouvrie
Hi, unfortunately I am unable to open the additional links to this study to check the conclusions that are drawn. To my humble opinion it must be a highly speculative outcome, because there are too many (unknown) factors. If herd-immunity could have ran it's natural course, the pandemic possibly would have been halted weeks ago. Now the virus spread is only slowed down. Vulnerable groups are still not safe. My guesses are if we had done a lockdown only on these groups, we would have been much better off. Now we face economic and social damage on a hughe scale, with doubtless more deaths and financial and social psychological problems upfront.
Based on figueres in Brazil, where there is no lockdown present, the current deaths are around 37.000 (09/06/2020) on a population of more than 212million inhabitants with very poor healthcare regarding to Europe. Deaths in Europe are 2 to 3 times higher with a lockdown. (probably due to unhealthier live styles and a more aged population). Or maybe all these figures are also based on specutalive guessing. Counted deaths having covid-19 in their bloodstream does not mean they have died from it, as we have many traces of virusses in our bodies all of the time. It is obvious the virus unmasks underlying diseases to these patients, who probably would have died next flew season in september or of something else.
Where can I find the complete documentation?
Josh Samuel
The authors have taken a very interesting approach to an important problem, but is there a risk that the choice of source data and use of piecewise constant modelling for Rt, combine to seriously inflate the impact attributed to full lockdown?
1. Choice of data - consistency across countries: I understand the authors preference for one central source of data, but I'm afraid that ECDC data, whilst held in one place, is not necessarily consistent. There is great variation in whether and when countries have included non-hospital deaths in the reporting, and whether all probable covid deaths are included, or just those with a positive test.
2. Choice of data - reporting delays: More importantly the ECDC data is based on date of report, not date of death. There are therefore significant delays between the date of the actual deaths and the date they are attributed to in the data. For the UK, for example, comparing date of death data to ECDC data, suggests around a 7 day delay in the ECDC data. It is not clear that the authors have accounted for this. It is also likely that this will vary by country. This will tend to push changes in R to seem later than they were, and thus push them towards the last intervention (usually full lockdown). Have the authors looked at modelling day of death data for hospital setting only? This would seem the "cleanest" data set to use, with the clearest link between community infection and time of death.
3. Use of piecewise constant for Rt: As the authors acknowledge, relying on modelling Rt as a constant allowed to change only at moments on policy intervention is somewhat artificial. This could nonetheless be a useful exercise, but if the reporting delay in the data pushes changes in Rt later in the series, then this will tend to inflate the changes attributed to the last intervention - usually full lockdown. The Sweden model, where full lockdown did not happen, is suggestive of this problem, with the the bulk of the Rt reduction there being attributed to last intervention, which in that case was banning of public events. Something that had nowhere near that impact in other countries. Also, fig 19 in the supplementary material shows that all interventions carry similar R reductions if modeled individually (this is not surprising, but shows how the piecemeal modelling struggles to attribute effects with interventions so close together).
It would be interesting to try applying the model to hospital date of death data for different countries, and seeing how sensitive the model is to different estimates of infection to death timing. Perhaps that could give a better indication of which interventions really did achieve the most impact?
Arun Thirunavukarasu
There are massive issues with this study, and I predict it will be retracted within the year:
a) Assuming that *only* lockdown and no other public health measures affect R is a fallacy.
b) Simulation data is used to argue against real evidence infection rate fell before lockdown.
c) Examples where identical results occurred without lockdown (e.g. Sweden) are ignored.
I'm disappointed that it didn't occur to the researchers or the reviewers how absurd the curves are - do they really believe that infections halted upon lockdown with no prior decline?
claude Replied to Arun Thirunavukarasu
Sweden is a bad example, since of its low testing rate (severely affected patients tested only) and because of its higher excess mortality (see: https://www.euromomo.eu/gra... in comparison to all other sparsely populated Northern European countries such as Norway, Denmark and Finland.
Arun Thirunavukarasu Replied to claude
Your selective comparison to other nordic countries and not for example, Spain or Italy, is telling - it seems you accept that factors other than lockdown are more important in the epidemiological trajectory!
claude Replied to Arun Thirunavukarasu
I'm sorry, I've been too quick to answer first. Yes, I agree, that the "complete lockdown" doesn't seem to be most important measure. Population density probably seems to be one essential parameter as well among banning big events and others. The impression of the Swedish infection process, however, to comparable neighbouring countries seems to hint on less efficient measures being taken. Still, a complete lockdown seems to be one brick in controlling the pandemic outbreak.
Arun Thirunavukarasu Replied to claude
This study attempts to claim that complete lockdown was the only significant measure reducing infection rate. Since infection rate peaked before lockdown measures in many countries, and Sweden experiences an almost identical rise and fall to other countries, it is obvious to me that these claims are absurd. Just look at the figures in the paper; they look absolutely nothing like reality - do these people truly believe R remained very high until (and only until!) lockdown was announced? Completely ridiculous - I hope this paper will be rescinded soon.
mosmosmos Replied to Arun Thirunavukarasu
Call me cynical, but given that this publication has been across the world media as 'lockdown has saved 3m lives', with very little critical analysis, it almost seems this was designed to get out ahead of anything which would show what an unnecessary action a 'full lockdown' was.
By the time it has been reviewed and retracted, it will barely be mentioned outside of the scientific community.
The data laid out in front of you in the paper just plain contradicts the conclusions drawn.
Nicholas Lewis
A major issue with the authors' approach is that other factors than non-pharmaceutical interventions (NPI) may account for most of the reductions in Rt. Obvious such factors are: unforced changes in behaviour, growth of herd immunity (particularly in large cities and other locations with a high Rt), and seasonal factors. Many NPI were adopted by all the countries studied. However, Lockdown - which the authors estimated to be far and away the most effective NPI (Figure 2) and the only individual NPI with identifiable effects - was not implemented in one country, Sweden. Therefore, Sweden is the only country studied for which the confounding effects of other non-NPI factors can, at least to some extent, be avoided.
In the original version of this study (Imperial College COVID-19 Response Team - Report 13), published on 30 March, the same day that the authors' manuscript was submitted to Nature, the date on which public events were banned on Sweden (12 March 2020) was correctly reflected in their plotted results, and after the final NPI of school closures some days later their central estimate for Rt was 2.6, with a 2.5% lower bound of 1.5. In the study's model, interventions have an immediate, step effect on Rt, so no subsequent further change in Rt was projected. The implication of this is that deaths in Sweden would continue to rise strongly, as they were projected to do in the short (early April) projection period of Report 13, until the epidemic engulfed most of the population. As we now know, that didn't happen; after mid-April Swedish COVID-19 deaths ceased to increase.
In the revised version of this study published in Nature, Rt for Sweden (Extended Data Fig. 1) is estimated to behave very differently, falling from 2.6 to 0.7 on 29 March 2020, with the result that subsequent recorded deaths are reasonably matched by the NPI-based model projections.
What caused this major reduction in estimated Rt on 29 March? Both Extended Data Fig. 1 and 4 show an intervention in Sweden on 29 March, being "Public events banned". But this appears to be just an invention by the Imperial College authors. Public events were actually banned from 12 March, as correctly stated in Supplementary Table 2 and the reference cited therein, and in the original (Report 13) version of the study.
As a result of inventing an imaginary Swedish NPI on 29 March, the authors' model projects a time profile of Swedish COVID-19 deaths that matches quite well the incidence of actual deaths (Extended Data Fig. 1). If the ban on public events were moved back to its true date, 12 March, the match between the time-profiles of projected and recorded deaths would be much poorer.
Even if the authors instead attributed the large modelled reduction in Rt to the previous NPI reflected in Extended Data Fig. 1, being 'Social distancing encouraged' on 16 March (the unmarked 'School closure ordered' NPI on 18 March had no evident effect) , would not solve this problem.
Altering the date of an NPI from its previous, correct, value with the result that the model projections well match recorded deaths in Sweden in itself destroys the credibility of the study. There are other very serious flaws in this paper, but the alteration of data casts doubt over the validity of the whole study. If Nature values its reputation, it would in my view be wise to insist on a retraction of this paper.
Nicholas Lewis Replied to Nicholas Lewis
Since posting the above comment I have realised that although the Timeline of Interventions (Supplementary Table 2) for this paper shows the date of "Public events banned" for Sweden as 12 March 2020, with events of >500 people banned, which is correct, on 29 March 2020 Sweden reduced the size limit of permitted events to 50 people. So there is a partial justification for the authors having treated the public events ban for Sweden as occurring of 29 March even though their own Timeline of Interventions table only gives the 12 March date and does not mention the modification of the ban made on 29 March.
JeiPi.
The authors wrote that "Most interventions were implemented in rapid succession in many countries, and as such it is difficult to disentangle individual effect sizes of each intervention. " jet they claim that only lockdown has an effect.
Pragmatist_in_another_guise Replied to JeiPi.
Boris Johnson picked his snake-oil salesmen and now we all have to live with the consequences.
Maurizio Rainisio
If the lockdown would have been as afficacious as claimed, lifting it (in Italy now for longer than 4 weeks) would have caused some kind of visible effect.
The Generalized Logistic that I fit to the Lombardy data, did not change a bit after early May. R2=0.999. https://uploads.disquscdn.c...
Pragmatist_in_another_guise Replied to Maurizio Rainisio
That is a chart wondrous to behold.
They key point is of simple statistical summary of the data revealing much the same as complicated 'black box' and 'take on trust' attempts mechanistically to model disease progression.
Jeremy Hooper
The modelling in this paper seems to stretch the bounds of reality and is not in line with previous modelling. The modelling undertaken by Imperial under the leadership of Prof. Fergusson suggested there were be around 550,000 deaths in the UK over a 2 year period if there was no mitigation strategies. This modelling assumed a death rate for Covid19 infections of around 1%, which is borne out by the current numbers of deaths and ONS estimates of actual infections.
The authors of this modelling suggest that there would have been around 500,000 deaths in the UK by the middle of May this year with the upper confidence interval suggesting it could have been as high as 600,000. This would suggest either a very much higher death rate than expected or that the entire population would have been infected in around a month. Neither seem likely in reality.
Further the modelling does not take account of the excess deaths which are non Covid-19 related which currently is at least 14,000. With more to come in the years ahead as the NHS attempts to get back to normal.
Its a shame the authors chose a sensationalist approach rather than looking to develop a balanced approach looking at the impact of Covid-19 infections and the measures taken to reduce the spread of the virus. Looking at Years of Life Lost approaches rather than number of deaths would be better and more balanced in its approach.
Pragmatist_in_another_guise Replied to Jeremy Hooper
Spot on, just considering deaths attributable to Covid-19 takes no account of morbidity and mortality arising from control measures like lock-down and from subsequent economic malaise.
Indeed, years of life lost analyses would shed valuable light. Unfortunately, mishandling by the government and gleeful talking up of disaster by news media has led to an emotional approach wherein each death is an equal and, supposedly, avoidable tragedy.
claude
Maybe of interest, since these publications show modeling of nonpharmaceutical measures, too:
https://science.sciencemag....
https://www.medrxiv.org/con...
Andrew Shardlow
Why bother with R?. Rather like a broken clock, we only really know it when it is 1. We don't have enough information to squeeze in time. A sensible approach is to focus on daily growth. Daily growth was tumbling down early in the course of the pandemic in each country studied and this fall pre-dates any NPI. There is no clear NPI impact on this, though there are two significant and curious yet consistent discontinuities seen in all daily growth factor vs. normalized mortality plots, including that from Sweden where there has been no lockdown. Sweden has a lower mortality per million than Spain, Italy, the UK and France. Your findings seem rather fanciful and there is insufficient detail in your paper to test the validity of a complex mathematical model in software in which there are probably many assumptions. The near straight lines segments in daily growth vs. death mean the time series data can be very precisely modelled simply by two logistic segments. https://uploads.disquscdn.c... The lockdowns may even have increased mortality.
https://uploads.disquscdn.c... https://uploads.disquscdn.c...
Pragmatist_in_another_guise
Did each of the many authors truly contribute to the analysis and its writing up? Given timescale for production of this work I doubt that practicable.
It would be far better, indeed less misleading, to distinguish the names of those doing the donkey work from others whose contribution was providing data and/or glancing over the penultimate draft.
Of course, the press and general public will be impressed by the authorship because to them it's obvious the more collaborators there be the greater the verity of that written (an extension of the fallacy of 'democracy').
-----
A nit to pick is: when did mathematics and computation become 'science'? Perhaps the authors don't make claim to being scientists but the press and government are under that illusion.
This is not intended to demean what they have done, there are plenty other grounds for that. Rather, it's a question of epistemological accuracy.
'Science' proceeds via the logic of refutation: predictions not borne out send a chain of (potential) falsification upwards to the originating theory/hypothesis. Mathematics is wholly deductive; its validation rests with peers in the field agreeing that the premises are not self-contradictory, and with the validity of each step in deduction; conformity with some external reality is irrelevant.
If the authors are engaging in 'science' then they have completed only the first stage of the process. Their model(s) loosely equate to theories/hypotheses. At best, they have attempted partial validation of their findings by reference to very recent measurable (with caveats) outcomes. Some of their predictions were notably off-track but this doesn't get mention.
To proceed with 'science' they could do the following. Refine their models (and assumptions) in light of what's recorded to have happened a few months down the line. Then re-run the model(s) and compare outcomes with observations. Keep adjusting the models (in somewhat atheoretic manner) until best possible predictions against current data are obtained. The next step is where scientific method emerges: test the model(s) in context of the next pandemic of comparable nature.
The upshot from the above is that everything produced by this team is currently speculative. It may be of great interest but nobody in their right mind would place these investigators 'at point' in leading a public health response. They may have valuable qualitative insights to offer but those ought be filtered through field-epidemiologists and public health professionals before being put in front of politicians. Seemingly, the Department of Health, and considerable expertise nationally and internationally the department can call upon, was sidelined when snake-oil was on offer. Perhaps somebody from the Department would care to tell me why I am under a misapprehension?
Joerg Stoye
For Germany, the paper gives a counterfactual *population* death rate of .68% (credible set up to .93%) by 5/4 and therefore a cumulative prevalence of 68%, and possibly 93%, by 4/20 or so. Does it sound plausible that Germany would have reached herd immunity and possibly asymptotic cumulative burden in that time frame? I tried but failed to understand the counterfactual's exact computation.
Dr Gareth Davies (Gruff)
From the paper:
"Our model relies on fixed estimates of some epidemiological parameters such as the [1] onset to death distribution, [2] the infection fatality rate..."
(Assumption numbering mine)
1) onset to death is not a constant - there's plenty of data to trivially demonstrate this - this assumption is invalid.
2) IFR is certainly not a constant and varies dramatically by location and most crucially by latitude as we have shown. This is also trivially demonstrated by considering the ratio of fatalities to recoveries which is an effective proxy for Hospital FR which is related to IFR by a fixed factor. More precise hospital data can be used to confirm this.
The two most fundamental assumptions underlying this model are demonstrably false.
jorgeplaza
A simple SIR model with constant parameters through the whole outbreak nicely reproduces the daily death rate in several cases, such as Spain as a whole, Madrid or New York. The key words are "constant" and "daily death rate". Constant, because there is no need at all to modify the rate of contacts in coincidence with the social distancing measures to fit the data. (Occam's razor: if a simple model with constant parameters fits the data, why should one assume that the contact rate changed in between?) Daily death rate because this is the only reliable piece of data: number of infections or infection rate are science-fiction and any model based upon them is suspect to me. If confinement was so effective, just by inspection of the daily death curves one should see a change of tendency in coincidence with the most extreme confinement measures, but this does not happen anywhere. The outbreak stopped because "the virus run out of susceptible people" due to the rampant rate of contagion (R0 as big as 7 or 8). I conclude the evidence in favor of the IC views is weak. They should explain first why nowhere in the world, with or without distancing measures, the infected share is greater than 20%. They should also explain the results of de Diamond Princess (less than 20% infected), to begin with.
Dr Gareth Davies (Gruff) Replied to jorgeplaza
I don't agree a simple model with fixed R and CFR fits the data. Not even for a single location like Spain. Do you have a link to such a model?
jorgeplaza Replied to Dr Gareth Davies (Gruff)
It's my model. Find herebelow the parameters for Madrid. The only reliable data, in my opinion, are daily deaths. Infections or infections per day are just science-fiction and I disregarded them.
number of effective contacts per infected person and day (beta) = 3,5415
rate of recovery (gamma) = 0,0586 / day
Madrid population = 6,7 x 10E6
Initial number of infected (february 1st) = 2,26E-02
Initial fraction of susceptible people, So = 11,3%
Lethality rate = 1,207%
As a consequence, Ro = 6,82
The model is sensitive only to the product beta x So; it yields essentially the same results if one assumes a double lethality and half as many susceptible people. I do not see any reason why 100% of the population must be allocated to the susceptible box. It seems to be so nowhere in the world.
For Spain as a whole:
number of effective contacts per infected person and day (beta) = 7,416
rate of recovery (gamma) = 0,043 / day
Spain population = 4,5 x 10E7
Initial number of infected (february 1st) = 8,18E-02
Initial fraction of susceptible people, So = 5,0%
Lethality rate = 1,367%
As a consequence, Ro = 8,62
I made a fit to daily deaths rate from feb 1st until mid may. Free parameters are beta, gamma, lethality rate and initial number of infected.
Check by yourself.
Dr Gareth Davies (Gruff) Replied to jorgeplaza
Thanks. I'll take a look. I agree detected infection numbers are meaningless. I've only modelled with fatalities and reported recoveries. The latter whilst less reliable than fatalities is still a good metric in early days since these come from hospital discharges.
You may find our study interesting (see link in my comment below).
Dr Gareth Davies (Gruff) Replied to jorgeplaza
Also if you Google "ESI Epidemic Severity Index" you'll see another technical paper on the technique I devised for this. I'd be interested to hear your views.
Sebastian Rosemann
Dear authors,
this study is an innovative approach using confirmed deaths as apparent manifestation of the pandemic.
But one major point is not clearly pointed out:
How valid are the modelled numbers compared to observations.
How does the non-uniform distribution of infections among age groups (that is observed in reality) affect the authors validation of their models?
Some details on this would be nice as otherwise all the numbers in this study are not provable by any real observation and therefore nothing more than imagined.
The explanation of how the age specific attack rates are calculated is hardly explained and very intransparent in the appendix with a lot of links and papers hard to find (e.g. Walker, P., Whittaker, C. & Ghani, A. The Potential Impact of COVID-19 in Lower-and Middle- Income Countries. ), i gave up to understand it. Same for usage and consequences for the rates in terms of the model.
As stated by the authors, the models are validated against results of e.g. antibody-studies in some countries:
„While there have still been few reliable national serological studies, initial small scale surveys in Austria and Denmark are closely aligned with our estimates.
A much larger study in Spain is very closely aligned with our estimates. These initial results, to some extent, validate our choice of infection fatality rate.“
These studies show different infection rates between age groups (see below).
So when a fatality rate is observed, this is a rate distorted by the age distribution of infections.
A simple comparison against the overall fatality rate seems not correct in most cases.
How do the authors deal with the age-distributions of real infections?
What is the exact measure to validate their modelled numbers against those studies?
Is there something like an age distribution deriveable from the models?
Some remarks to age distributions:
Spain:
https://www.ciencia.gob.es/...
~ 6,9% seroprevalence in age group 70-74 vs. ~ 5% overall
Belgium:
https://www.medrxiv.org/con...
~15% seroprevalence in the age-group 90+ vs. ~ 6% overall
Denmark:
https://www.medrxiv.org/con...
Only ages 17-69,
~ 2.3% seroprevalence in the age group 60-69 vs. 1.8% overall (17-69 years)
Austria:
http://www.statistik.at/web...
states that 0.15% of the austrian population could have been infected.
The only value that would roughly match the 0.76% in the authors paper would be the prevalence of 0.7% in risk areas (which is probably not all of Austria).
GeorgeDance Replied to Sebastian Rosemann
"How do the authors deal with the age-distributions of real infections?" I'm still getting into the new IC paper, but I'm at that point now. Using each country's demographics, they come up with a different IFR for each one. It's admittedly a gloss, as it doesn't take into account regional variations; but it is something they try to account for, not something they ignore (though of course it has been ignored completely in the media accounts - outside the stuff on Lockdown Sceptics, I haven't seen any media discussing the paper's methodology).
willem engel
1. correlation vs causality, it assumes without proving
2. big title always suspicious
3. The DATE! look at the that 30 march but with data from May? HIGHLY suspicious
Giuseppe Rotondo
Dear Authors
Death is such a variable parameter that cannot be used to build an universal model. Death regard a specific target of people i.e elderly with chronic pathologies. In Italy depended in elderly hospices contagions and so on..
Chris Horton
The article appears to present a somewhat circular argument. By first fixing a certain fatality ratio, then assuming that R changes only step-wise from an intervention, and also finding that lockdown is the only intervention having much effect on R, the model estimates that lockdowns drove R step-wise below 1 and averted a certain number of fatalities in each of the 11 European countries modelled. From this it is concluded that lockdowns had a large effect on R, and that continued intervention must be considered to keep R under control.
Nothing wrong with a circular argument, except there is little to stop doubts spreading from a single uncertainty until nothing is certain. For instance: in Extended Data Table 1, it is uncertain whether observed deaths in Sweden should fit the model or the counterfactual, when Fig 1 for Sweden models a step change in R as if it had locked down, yet Extended Data Fig 4 confirms Sweden as the 1 country of 11 which did not lock down.
What effects might this have on the assumptions and/or conclusions?
Nicholas Lewis
I have posted a detailed critique of the paper at my website: https://www.nicholaslewis.o...
I have run the model both with the authors' base assumptions and after
making slight changes to them. I find that the attribution of the reduction in transmission between different intervention measures is highly sensitive to the
precise assumtions made, particularly as to the delay between infection and
death.
Further, the Bayesian prior probability distributions used by the authors
for the parameters representing the estimated effects of each intervention are
extremely strongly biased in favour of finding that almost all the
interventions have a negligible effect, with almost the entire reduction in
transmission being due to one, possibly two, types of intervention.
Moreover, the authors make the highly unrealistic assumpption that each
country's population is homogeneous as regards both susceptibility to COVID-19
and infectivity. Moreover, they ignore other non-intervention factors that are
highly likely to have slowed transmission of the virus, such as unforced
changes in individual behaviour in response to the epidemic and seasonal
factors. In combination these result in their model being bound to estimate (a)
that almost the entire reduction in transmission was due to government
interventions and (b) that deaths would have been very high in the absence of the
interventions.
As a result of these shortcomings, the findings in the paper are unsound and
scientifically worthless.
Chris Horton Replied to Nicholas Lewis
Interesting as it is to pick over the dynamics of R, might that be both overcomplicating matters and looking in the wrong place? A circular argument must surely have but a single point of failure, from which everything else unravelled. It shouldn't be so hard to spot, nor too hard to repair.
Doubting the susceptibility and infectivity of populations, as per Lewis (2020), would seem to be getting closer, with its challenge to the unstated a-priori assumption that the Population Fatality Ratio must be the known Infection Fatality Ratio. A glance at Extended Data Fig 5 and perhaps we are there: assume a PFR, rather than the IFR, and re-fix the epidemiological parameters with a fatality ratio 10x lower (from distributions around 1% to around 0.1%). This step might re-align the counterfactual with Sweden, and relieve the model from the stress of its contradictions.
With the fatality rate re-fixed, it could no longer be assumed that R responds only to interventions. The model might instead assume that, with no intervention, R reflects a gradual approach to herd immunity. The model could then estimate the effect of each non-pharmaceutical intervention, in each country, on holding infections short of that eventuality, and where continued intervention should be considered to avert a renewal of transmission.
Ray Pawson
Every modeler is aware of George Box’s famous challenge: ‘the question you need to ask is not “Is the model true?” (it never is) but “Is the model good enough for this particular application?”’ The COVID-19 crisis has resulted in an epidemic of model building and the outpouring of simulations and extrapolations has had a profound effect on government policy the world over. Model building in all of its various guises relies on ‘estimates’ of infection parameters and it is the veracity of these assumptions that so vexed Box. Let us examine the two key assumptions built into Flaxman et al: i) ‘changes in the reproduction number are an immediate response to interventions rather than gradual change in behaviour’, ii) ‘individual interventions have a similar impact in different countries and that the efficacy of those interventions remains constant over time’.
These assumptions are plainly wrong. People react to lockdowns in dozens of different ways – they comply, they resist, they grow weary, they change their minds, they seek exceptions. For example, lockdown adherence varies: i) according to whether it relies on policing or persuasion, ii) with the degree of political, media and popular support for the measure, iii) on externalities as when protesters enraged at the George Floyd killing ignore the rules on large social gatherings, iv) on national temperament, which varies from high compliance to deep division, and above all, v) over time with resolve weakening as lack of contact hits home.
The same fluctuations apply to all NPIs. Nudge intervention are notoriously short lived – how many people still wash hands to the double rendition of ‘happy birthday’? Guidance is generally disputed even in the single community – reopening schools is interpreted either as an unwarranted risk (amongst those whose fear of infection persists), or a blessing (amongst those who perceive the threat has diminished). Finally, there is the ‘free rider’ problem, the disharmony provoked by people who believe their circumstances render them exceptions to the rules. These include government advisors and mathematical biologists.
Stefan Homburg
The article ASSUMES that the effective reproduction rate, Rt, is constant before and after interventions. Rt is allowed to change ONLY at the intervention points. This assumption drives the results and is at variance with the data. Using the same method, the authors could confirm a strong influence of sunspots on infections.
Jointly with Christoph Kuhbandner, I have written a formal comment that you can find on the Advance preprint server:
https://advance.sagepub.com...
xyzskaft
The previous three comments made me curious. Looking around online I found this interesting and valid critique of the methodology: https://www.medrxiv.org/con...
Some sort of disclosure: Both myself a and the authors of the work I found are Swedish. Biased or not, the methodology in the paper remains seriously flawed.
Michael Verstraeten
1.This article and the earlier reports of the same group have been used by several governments to “educate” the public about the severity of Covid – 19 in application of the International Health Regulations, the Report of WHO-China Joint Mission on Coronavirus Desease 2019 (Covid 19), 16-24 february 2020 and the following Strategy Updates published on the website of the WHO. For Belgium, the Minister of Internal Affaires referred to the earlier reports in the Ministrial Order (Ministerieel Besluit) form the 23the of March 2020 and the earlier and later Orders. The article itself was used by the Belgian state in a recent procedure to justify the Covid – 19 policy of the government stating that this policy has saved 120.000 lives in Belgium.
2.It has been recognised by the Belgian Governement (2° note to the Prime Minister from the GEES, 24.4.2020, p. 14) that fear for Covid – 19 caused deaths among the population: “… those who were severely ill at home but too afraid to seek care or not getting hold of the care they need. The latter 2 are particularly worrisome and have led to increased morbidity and mortality.”
3.This article and the earlier reports are based on the false assumption that the natural Rt of Covid – 19 is constant. For Belgium the natural Rt without lockdown in the period from the 1the of March to the 13the of March 2020, decreased from 2,2 at 1.3.2020 tot 1,22 at 13.3.2020 (Advise Celeval to the Prime Minister from the 27the of March 2020). The first lockdown measures were introduced the 14the of March 2020 at 00.00 h. In the current article, the evolution of Rt in this period is reported as constantly higher then 2. Which is FAKE. From the reports in The Netherlands, the same natural decline of Rt has been established in the report of the RIVM form the 14the of Juni 2020 (p. 26).
4.That Rt decreases in its natural environment is a phenomenon that is constant in all historical flu episodes. There is no historical evidence that ever a flu episode caused an infected population of entire countries let alone entire continents. Even not 60 % of their population. Levels of infection of this magnitude were only seen in clusters in a city, region or on a cruise-ship for instance. Reference to the Spanish flu dating from 1918 is not relevant since the situation of war was not comparable to the current situation (hygiene – general health condition of the population – medical care facilities). In the last 100 years, this kind of spread was never seen anymore. The Asian flu caused in the UK 17 % of its population to be infected. Normal flu episodes have an infection rate of 8 to 10 % of the population.
5.This is in fact what we see currently with Covid – 19. There is no country of a normal or bigger size that reports higher infection rates then 8 to 10 %. The surprise that Sweden reported also such a “low” infection rates was therefore irrational. As was its attempt to reach “herd immunity” of 60 %. All countries without lockdown report much lower death rates then the outcome of this article. Iceland, 8 states in the USA, Belarus… All of them got very high fatalities predicted, but in none of them this became reality.
6.In normal statistic sciences, assumptions are based on historical data. Even if the virus is new, the data of comparable viruses can be used. In case there is no historical data available that is comparable, assumptions become hypotheses and they need to the subject of the model themselves. In this study, the first step had to be to compare the evolution of Covid’s Rt to other flus Rt’s in order to check out if the Rt’s are comparable. One cannot assume the existence of E.T. before he landed on earth.
7.There is a very simple explanation for the existence of a correlation between deaths and governmental actions. Rt increases and decreases naturally over a classical flu episode of about 10 weeks. For Covid 19 the first patients start dying only after about 2 weeks. Government response will occur as early as in week 3 or 4, or even later. 2 weeks later, the natural evolution of the virus episode, being in the middle of the episode, will show a decreasing amount of daily deaths. So there will always be a time-driven correlation between the implementation of deaths and Government response. In Belgium the hospitalisation figures were growing less fast form the 21the of March on. Though the first lockdown measures date from the 14the of March, hence only 7 days before the moment the curve started to flatten. The time-driven correlation between declining death figures and governmental actions causes an acceptable confidence interval but does not establish a causa between them.
Being an MBA, I have a déjà vu with the banking crises now about 13 years ago. In that time a vast majority of statistical models proved to be fake. Assumptions with large but unknown margins of error were put into exponential statistical models, causing even bigger margins of error.
So if Nature and science wants to keep up a little bit of its credibility, we urge Nature and the authors of this freaky article, to withdraw it immediately before even more frightened people die. I’m sure that all your combined knowledge can offer the world realistic and useful models to extend the quality lives of our elderly brothers and sisters, within the limits of their own choices in their last months and years.
Michael Verstraeten, MBA, attorney at law.
Tom B Replied to Michael Verstraeten
https://uploads.disquscdn.c...
Verstraeten, do you really believe posting an internet comment on a website counts as "being published in Nature"?!
xyzskaft
Found another analysis well worth reading in detail:
https://judithcurry.com/202...
VERBATIM copy of conclusions:
Conclusions
First and foremost, the failure of Flaxman et al.’s model to consider other possible causes apart from NPI of the large reductions in COVID-19 transmission that have occurred makes it conclusions as to the overall effect of NPI unscientific and unsupportable. That is because the model is bound to find that NPI together account for the entire reduction in transmission that has evidently occurred.
Secondly, their finding that almost all the large reductions in transmission that the model infers occurred were due to lockdowns, with other interventions having almost no effect, has been shown to be unsupportable, for two reasons:
the prior distribution that they used for the strength of NPI effects is hugely biased towards finding that most interventions had essentially zero effect on transmission, with almost the entire reduction being caused by just one or two NPI.
the relative strength of different interventions inferred by the model is extremely sensitive to the assumptions made regarding the average delay from infection to death, and to a lesser extent to whether self isolation and social distancing are taken to exert their full strength immediately upon implementation or are phased in over a few days.
It seems likely that the inferred relative strengths of the various NPIs are also highly sensitive to other assumptions made by Flaxman et al., and to structural features of their model. For instance, their assumption that the effect of different interventions on transmission is multiplicative rather than additive will have affected the estimated relative strengths of different types of NPI, maybe substantially so. The basic problem is that simply knowing the dates of implementation of the various NPI in each country does not provide sufficient information to enable robust estimation of their relative effects on transmission, given the many sources of uncertainty and the differences in multiple regards between the various countries.
Arne Elofsson
It is very clear that the conclusions made are not supported. The use of a "last lockdown" feature for Sweden only seems to be necessary to achieve the results that the authors wants, see further analysis at https://www.medrxiv.org/con...
zeuszeus00
This paper is fraudulent and should be retracted.
Fundamental flaws in the analysis have caused false conclusions to be drawn. Correctly handled, the data actually showed the opposite: the lockdown had no effect on the course of the pandemic. These are the views of Dr Sucharit Bhakdi and Dr Karina Reiss in their book Corona False Alarm? (pages 60 and 61).